Innovations in the Indian Pharma Sector – The pitch for innovating

India’s Domestic Pharmaceutical Firms and Their Contribution to National Innovation System-building
Domestic pharmaceutical firms continue to operate under the influence of the strategy of global integration of the pharmaceutical industry and healthcare. The link between domestic firms and public sector research organisations is the weakest link in the domestic pharmaceutical industry due to misguided policies in competence-building and innovation system-building after India accepted the Trade-Related Aspects of Intellectual Property Rights Agreement in 1995. The government should rethink its strategies to get domestic firms to contribute to system-building activities and prioritise investment into the upgrading of processes of learning and building competence.
After India accepted the Trade-Related Aspects of Intellectual Property Rights (TRIps) Agreement in 1995 to remain structurally and systemically competitive, the domestic pharmaceutical industry needed to catch up with the advanced pharmaceutical countries in the development of in-house competencies and innovation system-building activities. Public and private investments were simultaneously required to be pursued for the creation of new knowledge or drug discovery, development of new processes, new formulations and dosage forms, new drug delivery systems and process intensification to technologically upgrade the domestic pharmaceutical firms and the national pharmaceutical innovation system. Contributions to innovation system-building activity also needed to focus on manufacturing excellence to remain viable and competitive in domestic and export markets simultaneously. Domestic pharmaceutical firms had to export to not only the markets of Russia, Africa, Asia and Latin America but also the highly regulated markets of the United States (US) and the European Union (EU), wherein they had to comply with the barriers raised in the form of regulatory and intellectual property requirements. In order to meet these requirements, their in-house research and development (R&D) efforts had to be geared up to file patents for non-infringing processes and formulations.
A highly liberalised regime of market access was emergent in the pharmaceutical sector since the domestic market had been opened up to allow the entry of foreign firms. Domestic pharmaceutical firms’ access to domestic markets was threatened due to the acceptance of product patents at home in several therapeutic areas. Funding support from the government for the implementation of collaborative R&D, to catalyse the active participation of domestic pharmaceutical firms in the development of new drugs for local health needs, was necessary. Context-specific steps to tackle the identified challenges were needed at the level of policymaking, institution-building, and system development for transformative change. Policymakers and domestic industry were required to act in a coordinated manner towards the development of supply- as well demand-side mechanisms to upgrade the national innovation system to meet all the identified requirements of public health and innovation.
India’s immature drug discovery system was full of structural holes arising out of the subcritical size of indigenous R&D expertise for almost all stages of preclinical and clinical work. A focus on the post-TRIps challenge of industrial and innovation system development required the domestic industry and the state to target the perusal of achievements of goals of structural and systemic competitiveness. Therefore, the Indian state’s priority was to create immediately favourable conditions, incentivise all the relevant actors appropriately to contribute to system-building and target structural weaknesses in existence on account of the small size of the domestic market. The structural weaknesses of the domestic pharmaceuticals market is due to the pharmaceutical sales of domestic firms being essentially dependent on out-of-pocket expenditure of the Indian population. The other structural weaknesses of the Indian pharmaceutical industry are the comparatively smaller firm size, the lack of specialisation with fragmented, over-diversified product portfolios even in relatively larger firms and the declining local production of active pharmaceutical ingredients (APIs). Pharmaceutical formulation manufacturing required considerable technological upgrading and strengthening in terms of quality control which was lacking in the Indian market.
This article examines the current state of India’s pharmaceutical innovation system in respect of all the relevant domains of pharmaceutical innovation, traces the journey of the last two decades to delineate merits and demerits of the pathways under formation, and analyses the sources of systemic failures and suggests possible solutions. The article argues that the post-TRIps pathways have failed to foster systemic and structural competitiveness in the pharmaceutical industry. The pathways of industrial and innovation system development came to be guided by the emerging policy path of neo-liberal globalisation. The approach to institution-building for the creation of a home market was heroic, unrealistic and conducive to the interests of foreign firms rather than strategically building the innovative enterprise that would have been capable of supporting the development of a self-reliant pharmaceutical industry.
This gap between the intent behind policymaking and the actual results was due to neo-liberal and market-driven pathways to industrial development and innovation system-building. Local production cannot be encouraged and public health problems cannot be addressed unless structural weaknesses are not tackled on an urgent basis. The article suggests that the post-TRIps pathways are in an urgent need of a radical transformation not only in respect of policymaking, but also in institution-building and system development to achieve the goals of structural and systemic competitiveness.
Drug Discovery, Research and Development
The current situation is that large domestic firms are unable to continue drug discovery with their own in-house efforts in India for almost any type of market need in respect of drug discovery and new drug development. Foreign firms are limiting themselves to exploiting the existing market and production and have no interest in the development of local technological capabilities.1 Analysis undertaken of R&D and patent portfolios of the leading domestic pharmaceutical firms at different points in time shows that it is not easy for the domestic pharmaceutical industry to continue simultaneously with innovation by making investment across all the relevant domains of pharmaceutical innovation (Abrol et al 2011, 2013, 2018; Abrol and Singh 2016a, 2016b, 2019).
Large domestic pharmaceutical firms have started to import their bulk APIs requirements from China wherein the domestic regulators do not go to even inspect manufacturing facilities (BCG–CII 2014; Department of Pharmaceuticals 2016). Large domestic pharmaceutical firms’ in-house R&D and innovation-making efforts continue to be embedded in the understanding that they mainly need the contributions of foreign acquisitions, alliances and collaborations and export markets and, thus, are satisfied with the enhancement of fiscal support for the strengthening of firms’ in-house R&D efforts (Abrol et al 2011, 2013; Abrol and Singh 2016a, 2016b). Differding (2017) also confirms that the post-TRIps pathways chosen were not good enough to sustain the competitive edge of domestic pharmaceutical firms in drug discovery.
During the post-TRIps period, domestic pharmaceutical firms had to invest in the building of technological capabilities to overcome the restrictions on their exports due to the emerging strategies of big pharma to delay the entry of India’s domestic firms into the US and EU markets by calling on stronger laws on intellectual property (IP) and much tighter drug regulations of the US and EU. In-house investment in chemistry-driven research processes got accelerated, leading to the development of several original non-infringing processes for APIs, the identification and characterisation of impurity profiling pertaining to APIs, the reduction of impurity levels, and acceptable dosage forms and formulations. In this context, Table 1 (p 35) elaborates the historical timeline of capability development profiles mapped by the authors on the basis of patents filed by the Indian pharmaceutical industry with the United States Patent and Trademark Office (USPTO) (Abrol and Singh 2016a).
The assessment made shows that during 2007–16, the efforts of domestic pharmaceutical firms remained focused on mainly the development of in-house R&D activity for the export of simpler generics to the regulated markets of the US and EU. Drug discovery and new drug development is on the decline. The focus of their in-house innovation-making activities is now essentially directed towards process development, new dosage forms, formulations, new drug delivery systems and technological know-how for off-patent generics. Efforts to develop the markets for fermentation and biopharmaceutical products were missing during the post-TRIps period. The bulk of “innovative outputs” belong to the areas of dosage/formulation/composition of matter and new forms of substances.
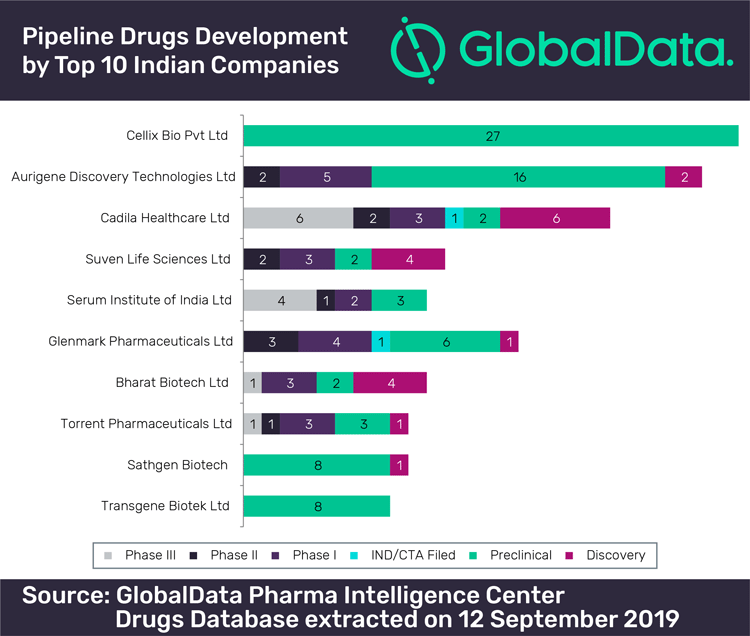
Initially, post the 2000s, drug discovery research drove innovation activity by stressing on in-house R&D capabilities for close to 30 domestic pharmaceutical firms (Differding 2017). However, the engagement of domestic pharmaceutical firms with drug discovery for new drug development has been on the decline from 2012. The number of patents filed on new chemical entities (NCEs)2 by the domestic pharmaceutical firms is small (Abrol and Singh 2016a). Presently, there are only three firms pursuing some activity in drug discovery and new drug development (Differding 2017).
New drug development-related inventive activity is still to emerge as a game changer in the creation of competitive advantage for the domestic pharmaceutical firms in India (Abrol and Singh 2016a; Differding 2017). Edmond Differding (2017) updated the assessment of the efforts of 28 major Indian pharmaceutical companies and of 14 biotech companies and start-ups that have reported proprietary NCE activity R&D with preclinical or clinical development compounds between 1994 and mid-2016. The analysis also shows that only 12 companies have engaged in long-term and large-scale drug discovery efforts and have produced a significant number of development compounds. For a majority of the companies, drug discovery remained a rather limited activity, as illustrated by isolated patenting of internal research results, or by academic collaborations which are analysed in the article (Table 6, p 35).
Weak In-house Capabilities
At the major pharmaceutical companies, many internal R&D efforts have not been met with the expected success due to weak in-house capabilities and a global market orientation of R&D where success is not easy. This can be seen from the R&D conduct of those companies that have abandoned drug discovery in the meantime, be it after being acquired, or before going public, or after failing to deliver commercial compounds despite significant investments (Differding 2017).
Even other companies, where drug discovery is still under perusal, have significantly reduced the number of NCEs in their pipeline, including Zydus Cadila (from 13 in 2011 to five in 2016), Glenmark (from eight in 2006 to two in 2016), and Sun (from five in 2012 to three in 2016) (Differding 2017). One significant exception is Lupin Pharma, with currently four compounds undergoing clinical development (Differding 2017). As an overall result, the combined pipeline contribution from a peak in 2011 with 58 compounds (89% of the total pipeline) decreased to 41 compounds (or 49% of the total pipeline) in 2016 (Differding 2017).
While a few contract research organisations (CROs) ventured successfully into proprietary research, the number of biotech companies and start-ups with proprietary compounds also remains small with 10 companies (Differding 2017). Currently, the increase in development compounds coming out of India is driven by R&D-focused biotech companies, with 42 compounds in 2016 (Differding 2017). These companies face significant constraints in developing and marketing their own drugs. These constraints include high costs of development, longer time horizons and lack of experience of clinical development. In particular, most small biotech companies have preference for out-licensing deals to finance part or all of their development costs. All of their relations with foreign firms are based on out-licensing deals or option agreements (Abrol et al 2011; Differding 2017).
Twenty-three small molecule compounds have been licensed to global pharmaceutical companies or have been under option agreements over the past two decades (Differding 2017).3 Deals entered into for drug development with big pharma by biotechnology companies do not aim to promote the objectives of local production and affordable access to medicines for the Indian population (Stevens and Huys 2017; Taylor 2016). There is a steady increase in the number of compounds for biotech companies. Out-licensing models of major pharmaceutical companies have experienced several resounding failures (Joseph 2012). A key observation from Thomas et al (2016) is that most of the out-licensed drugs by big pharmaceutical companies based in India are being returned back citing lack of sufficient competitive advantage (Thomas et al 2016).4
Currently, all major Indian companies report annual expenditure in the range of 5%–10% of revenues (Differding 2017; Joseph 2012). The NCE research is not more than 25% of the total R&D expenditure (Differding 2017). The overall investment in new drug discovery and development is close to about 2%, which is only one-tenth of the average expenditure on R&D of the US pharmaceutical industry (Differding 2017). The majority of Indian pharmaceutical companies have opted to spread their efforts over multiple therapeutic areas. Few Indian pharmaceutical companies have specialised in particular disease areas (Differding 2017).
The top three of the resulting 23 classes, that is G-protein-coupled receptors (GPCRs), kinases, and nuclear signalling pathways, represent about half of all targets. Few domestic companies have focused on specific targets. In general, companies adopted a broad approach with multiple target types (Differding 2017). The problem of lower success rates is compounded by the increased time spent at the earlier stages. This shows that the drug discovery process at Indian companies is considerably far less efficient than the industry average.
Manufacturing Innovation Performance
Estimates indicate that currently 50%–70% of drugs manufactured have import content (finished APIs or intermediates) and over half of this comes from China. The larger domestic companies are actually dependent (up to 70%–90% of volume manufactured) on the export market, and the domestic market is not growing. The “success story” of the Indian pharmaceutical industry will be over when China enters the finished products market globally, which it is starting to. Figure 1 (p 38) and Figure 2 (p 39) illustrate the important details of the extent of import dependence prevailing now in the case of the Indian pharmaceutical industry.
The conjecture that allowed the pharmaceutical sector to grow by importing raw materials from China for the production of formulations will not continue for very long. China is also developing its formulations sector for the promotion of exports to the US and EU. The post-2000s pathways need to shift from the pursual of the low road to industrial upgrading to prevent the domestic generic pharmaceutical industry from falling behind China. It is also clear that very few firms have come forward to collaborate with the Council of Scientific and Industrial Research (CSIR) institutions for the development of continuous manufacturing technology.5
The industry has been neglectful of several of the relevant objectives of manufacturing excellence. The related challenges facing the industry in respect of the cost of production, investment in plant and machinery, efficiency of power, fuel, and water use, and management of environment and pollution control have been poorly addressed by the industry (Abrol and Singh 2019). Table 3 lists the key indicators of performance in respect of the achievement of the goals of manufacturing excellence.
Furthermore, the changes being brought in the guidelines for regulatory compliance or in the inspection process by the US and EU since 2003 have also failed to get an appropriate system-wide technological and organisational response from the industry, R&D institutions, and the policymaking apparatus in India. When the post-2000s policy path was formulated in 2000, the challenge of the incorporation of the principles of continuous manufacturing was not high on the radar of the US and EU. However, within three years or so, from 2003–04 onwards, the US Food and Drug Administration (USFDA) had started inducing big pharma to invest in manufacturing innovation (Center for Drug Evaluation and Research 2014). Pre-competitive R&D plans and new collaborative mechanisms had begun to become the new pharmaceutical innovation strategy of the US. Similarly, the German pharmaceutical and fine chemical industry had begun to consolidate and restructure their manufacturing operations.
Structural competitiveness required the large domestic firms to focus on the upgrading of their API manufacturing function. The government was required to systematically attend to the upgrading of pharmaceutical manufacturing clusters in the western and southern regions of India to foster energy, waste and environmental management-related innovations in manufacturing. But the post-TRIps policies preferred to take the route of neo-liberal and unregulated path of cluster development. The pharmaceutical and allied industries cluster of Baddi in Himachal Pradesh is a prominent example of this path leading the industry to embrace a low road to industrial development in many other clusters as well during the post-TRIps period.
Structural Weakness and Systemic Competitiveness
Currently, domestic firms lack in the ability to invest in innovation system-building activity across all the relevant domains of pharmaceutical innovation simultaneously. Apart from the challenge of drug discovery for the development of new drugs, incremental processes and product innovations, domestic firms are also faced with the challenge of the development of manufacturing capability to serve domestic and export markets simultaneously. Manufacturing regulation framework is being tightened in the US and EU as well as in India. The ability to invest in innovation-making, across manufacturing, processes and products simultaneously, and obtain returns from these activities depends primarily on the size of the home market that domestic firms can easily access and link with to undertake successful system-building activity.
New structural weaknesses are emerging on account of the lack of a commensurate growth of the domestic market due to the continued dependence of the domestic firms’ sales on out-of-pocket expenditure of the Indian population, the inability of domestic firms to move to product specialisation due to the dependence on fragmented, overdiversified product portfolios, and the imbalances developing in the product portfolios due to highly skewed dependence of large firms on the exports to regulated markets of the US and EU. Apart from these problems, in-house local production of APIs is declining, there is a lack of attention to data integrity in pharmaceutical formulation manufacturing, and a weak organisational innovation to improve quality control and a weak pharmaceutical regulatory framework and apparatus are the other emerging structural weaknesses.
These emerging imbalances in the innovation portfolios are worrisome trends and should be a matter of concern, as our analysis reflects new structural rigidities and institutional voids. The neglect of manufacturing innovation can adversely impact the capacity of domestic firms to invest in the development of in-house capabilities for both production and innovation even in the short and medium terms. During the post-2000s period, the pathways chosen for industrial development and innovation-making did not prioritise the goals of structural and systemic competitiveness and pursued a low road to achieving the goals of cost competitiveness.6
During 1997–2006, the contribution to income earned by domestic firms from the expansion of the domestic market had already reduced to close to 50%. During 2007–16, the growth rate of domestic sales for large firms declined even further. In a significant way, the contribution to income from the expansion of the domestic market came down to almost 35% (34.97% to be precise). During 1997–2006, the contribution from the income earned from the expansion of export market for domestic firms was about 22%. During 2007–16, this share increased to 34.44%. Over 55% of India’s exports are to regulated markets.
The domestic market grew far more slowly than the export of goods and services in the Indian pharmaceutical industry during 1996–2016. Table 4a charts the changing pattern of reduced domestic market sales and the doubling of the share of export of goods and services. During 2007 to 2016, exports grew rapidly in comparison to the growth of domestic sales. Table 4a brings out how the strategies of market development emphasised the export of generic pharmaceuticals (export of goods), and the supply of contract manufacturing and research services (export of services) to foreign firms.
In the post-2000s period, India’s policymakers went on to promote an imbalanced growth of sales revenue in the case of large domestic pharmaceutical firms in an accelerated and highly skewed way. Large firms were allowed to grow by increasing their sales revenue through the expansion of pathways of low road to industrial development in five ways. First, large firms were allowed to produce and sell branded generics and combination products in the domestic market. Second, large firms were allowed to outsource production to small-scale firms for sale in the domestic market. Third, domestic firms supplied contract manufacturing and research services to foreign firms. Fourth, large firms were allowed to build strategic alliances and collaborations with other large firms of domestic and foreign origin. Fifth, trade was liberalised with a view to encourage exports embedded in imported active pharmaceutical ingredients (Abrol and Singh 2019).
Consequently, there has been a rise in the number of large firms who do not undertake in-house manufacture of the core ingredient of the pharmaceutical formulations, or APIs, by themselves. That has made India dependent on China for key starting materials (KSMs), intermediates and APIs (Figures 1 and 2). Large domestic pharmaceutical firms are being allowed to use the pathways of loan licensing and contract manufacturing. All of these steps are only encouraging the Indian industry to remain a low-cost producer and exporter of generic pharmaceuticals to the regulated markets.
The pharmaceutical industry is moving away from the practice of undertaking manufacturing for self (Table 4b, p 39). Evidence suggests that sales in the domestic market are now increasingly taking place via the pathway of manufacturing through others. The contribution of pathway of manufacturing for self or in-house declined rapidly from 69.74% in the first decade (1997–2006) to 38.69% in the second decade (2007–16) in respect of the domestic market. Computed as export of services, the share of contract manufacturing of APIs and formulations for foreign clients rose from 1.08% in 1997–2006 to 14.64% in 2007–16.
Analysis shows that the domestic pharmaceutical industry has not succeeded in pursuing the pathways capable of overcoming the structural weaknesses arising out of the underdeveloped nature of linkages and complementarities of the national innovation system, the small size of the domestic market, and the continued weakness in the in-house technological capabilities for innovation-making across all domains. Even today, after two decades, the pockets of the domestic pharmaceutical industry are not deep enough to sustain innovation system-building activity for the perusal of innovation-making activity simultaneously across all the relevant domains of pharmaceutical innovation. In fact, there are now a large number of new structural weaknesses arising on account of a highly skewed pattern of market development and in-house manufacturing capability development of large as well as small pharmaceutical firms.
Pathways of Global Integration and System-building
The most important element in the post-2000s policy path was the contribution of alliances and collaborations with foreign firms and support from international non-governmental organisations (INGOs) for system-building. The assumption was that national innovation system-building would be accelerated with the help of strategic alliances and collaborations of domestic firms with big pharma. The globalisation of R&D and innovation activity was seen as an emerging beneficial trend. The domestic pharmaceutical industry was asked to embrace this emerging trend without any kind of safeguards and
caution (Chaudhuri 2014).
The government gave liberal tax concessions to domestic pharmaceutical firms for the promotion of exports to regulated markets. While these tax concessions were available to the domestic firms, they did not have to commit themselves to the fulfilment of performance requirements for manufacturing excellence or development of domestic market and local in-house production of APIs as an obligation. The fiscal support did not carry any conditionalities and therefore, it is not surprising that existing structures of India’s national system of innovation are inadequately developed for the next phase of challenges arising in the upgradation of pharmaceutical manufacturing (Abrol and Singh 2019).7
Even today, the investment of CROs that offer chemistry- and biology-based R&D services related to drug discovery from the Indian soil is a much smaller effort compared to China. While the Indian government aims to stimulate the launch of 2,000 start-ups in the life sciences over the next five years, it is unlikely that most of these start-ups will venture into new drug development and drug discovery based on their own proprietary NCEs. Although smaller research-intensive biotechnology companies specialising in niche areas of expertise with their focus on specific disease areas or target classes are doing better compared to the established pharmaceutical companies, their capacities are dedicated to provide services to big pharma (Differding 2017).
The path of integration of the capabilities of domestic firms with the existing networks of global production and innovation (GPINs), dependent on the development of contract manufacturing and research services by big pharma, has not worked for the benefit of system-building and industrial upgrading. Strategic alliances and collaborations are focused far more on the development of marketing rather than on manufacturing and R&D functions. Their existing capacity to commercialise the technological knowledge for the benefit of local production and drug discovery is very weak.
Therapy-wise differences in the ability of foreign or domestic firms to use the patent as an instrument of market control for the establishment of market monopoly, market duopoly, and market leadership exist. Foreign firms have been able to obtain a significant market share in respect of the products in use for the treatment of diabetes, cancer, cardiac issues, urological issues, problems of the central nervous system and rejuvenation, which fall in the category of acute and chronic conditions. Their sales are growing rapidly. The competitive advantage of foreign firms is on the rise (Abrol et al 2016).8
Domestic companies happened to be market leaders in close to 84% of the products approved before 2005. Foreign companies were market leaders in only 16% of the products approved before 2005. Today, foreign firms are market leaders in 38% of the products that contain APIs approved after 2005. In the sales of 91 compounds out of 268 compounds where the concentrated market structure has emerged, foreign firms are market leaders in 23 compounds out of 30 post-1995 compounds (Abrol et al 2016).
There is a need to keep a close watch on future developments. Domestic industry and publicly funded R&D institutions need to get together to develop a joint strategy to keep a watch on the patenting of NCEs and the landscape of clinical trials in the making. The growing share of patented NCEs by foreign firms in the Indian patent office should be a matter of concern in the light of the changing composition of the domestic retail market for the consumption of newer medicines in respect of non-communicable diseases.
There are 877 NCEs wherein foreign firms are dominating the emerging landscape of pipeline-patented compounds. The pattern of concentration of ownership is a cause for worry; the distribution of patents granted for NCEs to Novartis (84), Roche (76), Sanofi-Aventis (73), AstraZeneca (62), Bayer (58), Pfizer (53), Boehringer Ingelheim (46), SmithKline Beecham (48), Aventis (44) and Janssen (39) is evident. A large number of NCEs patented for the compounds targeting anti-inflammation, heart diseases, anti-depressants, cancer and respiratory diseases by foreign firms are in the pipeline.
Furthermore, calculations also show that close to 4% of the total sales of 2015 can be attributed solely to the positive impact of patent oppositions on access to medicine in India. The well-known examples are of two important post-1995 products, namely Novartis’s anti-cancer drug, imatinib mesylate, and Gilead’s anti-HIV/AIDS drug, tenofovir disoproxil fumarate. There are also other kinds of patent oppositions that continue to play a positive role in the reduction of market power. In the case of Darunavir, successful opposition mounted to a patent application on the method for the synthesis of an intermediate of Darunavir (Prezista) played a positive role. It was rejected under Sections 25(1)(e),(f), and Section 3(d). Rejection was mounted in the case of Gefitinib under Sections 25(1)(e), 3(d), and 2(1)(j). In the case of Adefovir Divoxil rejection came under Sections 25(1) (e), 3(d), and 2(1)(j).9
Domestic firms have been able to emerge as the market leaders in the case of many important molecules only through patent oppositions; there is the possibility of the competitive advantage of domestic firms eroding for those compounds where many of the patented NCEs are already under clinical trials in their third phase in India. Foreign firms prefer to import medicines whose product patents are strong and enforceable. The favourable impact of delayed TRIps implementation is largely on account of the successful public opposition put up to the early introduction of pharmaceutical product patents in India.
Price Control of Patented Medicines
India needs to use the standards of full disclosure (enablement), novelty, inventive step, and industrial application to keep out the trivial inventions from being patented in India. India also needs to use the compulsory licensing provisions for commercial use, public interest, and government use. This will help bring down the share of emerging product monopolies. India lacks a policy for the price control of patented medicines. There is evidence that the new patented drugs are being marketed at exorbitant prices for life-threatening diseases such as cancer, cardiac diseases, diseases of the central nervous system, nephrological diseases, diabetes, and hepatitis. Some of these medicines have been introduced through
marketing arrangements established with the help of domestic pharmaceutical firms.
The rise of oligopolistic competition is evident in a larger number of compounds in 2015. Emergence of the monopolies in the case of 31 out of 60 compounds accounting for 50% of the post-1995 compounds is an important development. Policymakers cannot ignore a fourfold increase in the sales of compounds experiencing oligopolistic market competition. Patent-enabled monopolies grew faster after 2005 in the Indian pharmaceutical industry. The rise of the sales of foreign firms for the compounds approved after 1995 is visible in the rising market concentration as well as in the share of patented compounds in selected therapeutic groups (Abrol et al 2016).
Weakening of the Public Sector
After India accepted the TRIps Agreement, policymakers deliberately pushed the public sector enterprises into a situation of no return. Policymakers thought that private investment of large domestic firms as well as strategic alliances and collaborations would do the job of industrial upgrading in a better way. The Indian pharmaceutical industry chose to move away from the path of development of indigenous raw materials. See Table 5 (p 40) for an overview of the performance of the five public sector companies over the last two decades.
All the changes made in the sphere of policymaking vis-à-vis the development of public sector have also had an adverse impact on the development of in-house competencies in private sector firms. Weakening of the contribution of public sector firms to system-building activities is also responsible for the weakening of pharmaceutical manufacturing in the last two decades.
It is to be noted that the challenge of upgrading in-house competencies of private sector firms was addressed during the pre-TRIps period through the development of the public sector in India. Scholars and policymakers recognise the contribution made by Indian Drugs and Pharmaceuticals Limited (IDPL), a public sector enterprise, and by CSIR laboratories to the development of in-house competencies of private sector firms working in the Indian pharmaceutical sector.
Emerging Challenges
The need of the hour is to strengthen urgent and need-based research by the public research institutions and the domestic industry working jointly on the substitution of imported APIs, intermediates and KSMs. Policymakers must convince the large domestic firms to concentrate their efforts on strategies that will free them from getting into dependent relationships with foreign firms. Most of the emerging Indian pharmaceutical multinational companies are not leveraging government funding for undertaking in-house R&D (Abrol and Singh 2019). Large domestic firms accounted for just 15 projects in the portfolio out of the 104 sanctioned by the government during 2000 to 2011 (Abrol et al 2013). Although they are not utilising the existing schemes in a systematic way, it is also true that the industry has been complaining about the rather small amount of government funding available for the direct benefits of R&D for the industry (Abrol et al 2013; Abrol and Singh 2019).
Domestic firms have not come forward to use government schemes for R&D and the innovation of therapeutics for tackling priority diseases. This is the case even when the science agencies are agreeing to cede the ownership of intellectual property rights (IPRs) to collaborating firms. It is obvious that there is a lack of interest among domestic pharmaceutical firms in the schemes under promotion by the government for drug discovery R&D. Their pockets are not deep enough to continue with drug discovery for global markets and they have also been unable to sustain their interest in drug discovery R&D. Some of these firms have now been sold by their promoters to foreign firms (Abrol et al 2013).
It is also clear that the national links of these firms are only getting weaker instead of becoming stronger. Universities, medical colleges and hospitals are yet to emerge as a key factor in the processes of building of a national system of innovation in India. It is not possible to take advantage of the biotechnological revolution without integrating the structures of clinical research, academia and industry (Table 6).
Pharmaceuticals as a ‘Strategic Sector’
The pathways chosen remain embedded in the heroic and unrealistic vision and directions formulated in 2000 in the Pharmaceutical Research and Development Committee, headed by R A Mashelkar, the director general of CSIR (Ministry of Chemicals and Fertilisers 2001). In this report, Mashelkar established five “gold standards” for pharmaceutical companies for availing themselves of exemption from the Drug Price Control Order. Although only a part of the policy got implemented with regard to the price exemptions, the measures sought to be implemented were heroic in conception, particularly on the aspects of learning, competence-building, and innovation-making. The report stipulated that there needed to be a minimum investment of 5% of the total turnover in R&D, employment of at least 1,000 scientists, a turnover of at least ₹ 10 crore, registration of at least 10 patents, and Current Good Manufacturing Practice (cGMP) approval for manufacturing facilities.
Drug discovery for local public health needs and competitive local pharmaceutical production requires the helping hand of publicly funded R&D (Sampat and Lichtenberg 2011; Stevens et al 2011). Pharmaceutical innovation system building efforts face an uphill task; all the policymakers’ expectations being belied completely with regard to the contribution of foreign firms to India’s pharmaceutical R&D and manufacturing landscape are clearly visible (Chaudhuri 2014). The fiscal and non-fiscal support measures should focus on the establishment of an autonomous Drug Development Promotion Foundation (DDPF) to promote R&D, public venture capital-based financing of young firms and mandatory collection of 1% of the maximum retail price (MRP) of all formulations sold in the country and a pharmaceutical R&D support fund.
All the recent analyses undertaken of the draft Pharmaceutical Policy, 2017 confirm that the policymaking establishment is unable to come out of the pathways based in a completely flawed understanding of how drug discovery and drug development has occurred in the past, even in the developed countries. For example, the Federation of Medical and Sales Representatives of India’s (FMRAI) submission on the draft Pharmaceutical Policy, 2017 critiques the policymakers’ understanding that the public sector is not essential, that the ease of doing business index of the World Bank is more important, foreign direct investment (FDI) policy can continue to allow brownfield investments and that patented drugs can remain outside price control and compulsory licensing mechanism (FMRAI News 2017).
Inevitably, national manufacturing and technology upgrading strategies need to become strategic in intent. The central government’s decision to allow up to 74% FDI as brownfield investment in the domestic pharmaceutical companies for their acquisition by foreign firms through the automatic route has serious implications for the development of pharmaceutical manufacturing capabilities and public health in India. Policies for industrial development and innovation-making have been discouraging the sector from pursuing the high road to industrial upgrading. Fortunately, the challenges arising out of the growing dependence on critical imports from China is an important security concern of the government as well as of the domestic pharmaceutical industry. The domestic industry can continue exporting generic medicines only through the development of clusters for fermentation products, critical starting materials, intermediates and active pharmaceutical ingredients, fluorination and biopharmaceuticals.
Conclusions
When the domestic firms took to the challenge of increasing exports as a priority in 2002, the post-TRIps pathways did not systematically focus on the critical aspects of developing the home market, creating local champions and strengthening the national innovation system. Rather, the post-TRIps pathways failed in building a self-reliant national system of production and innovation. The structural weaknesses continue to persist and need to be overcome through the development of appropriate pathways on both the supply as well as demand side.
Today, with the consolidation taking place in the US, pharmacy chains based there have improved their bargaining power and there is contracting of the margins of the Indian exporters (Trivedi 2017). As their revenue gains remain significant from the export markets, domestic firms are looking to newer options. Domestic firms are yet to make their presence felt in the manufacture of complex generics, specialty products, biosimilars and other innovative products (Chandna 2019). Domestic pharmaceutical firms are at a crossroads in the wake of the US and EU governments gradually tightening their regulatory landscape to protect their own firms’ competitive advantage worldwide.
As things stand, it is also apparent that for a major success in drug discovery, India will have to wait for the pharmaceutical innovation system to gain maturity. In the absence of steering and coordination of the outward foreign direct investment (OFDI) connections, the domestic pharmaceutical firms are not able to upgrade the national system of innovation through foreign alliances and collaborations. The conduct of domestic pharmaceutical firms is not qualitatively different from the firms originating from the developed capitalist world. That domestic firms have preferred to enter the markets of Africa, Asia and Latin America through wholly owned subsidiaries rather than joint ventures needs to be recognised as an emerging political concern for India’s international relations with these regions. Policymakers will have to strategically target the challenge of export and domestic market-building, deal with information externalities arising out of a weak institutional research base, and remedy the coordination failures in respect of the promotion and regulation of technology development. The challenge of technological and industrial upgrading has been on the door step of Indian policymakers for quite some time. Domestic firms can build their competencies in collaboration with publicly funded R&D institutions and public sector industry.
The Indian policymakers have a social responsibility to ensure that the institutions of health sciences remain geared to producing more of public goods than market goods. In particular, they have a duty to use the instruments of public sector R&D and government support for innovation to the private sector in a targeted way. The private sector would not be able to stay in the business of pharmaceuticals without help from the public sector. At the moment, the introduction of a strong public sector is a necessary condition for the systematic development of the private sector in both manufacturing and R&D.