US CDC recommends Novavax’s COVID-19 vaccine for adults
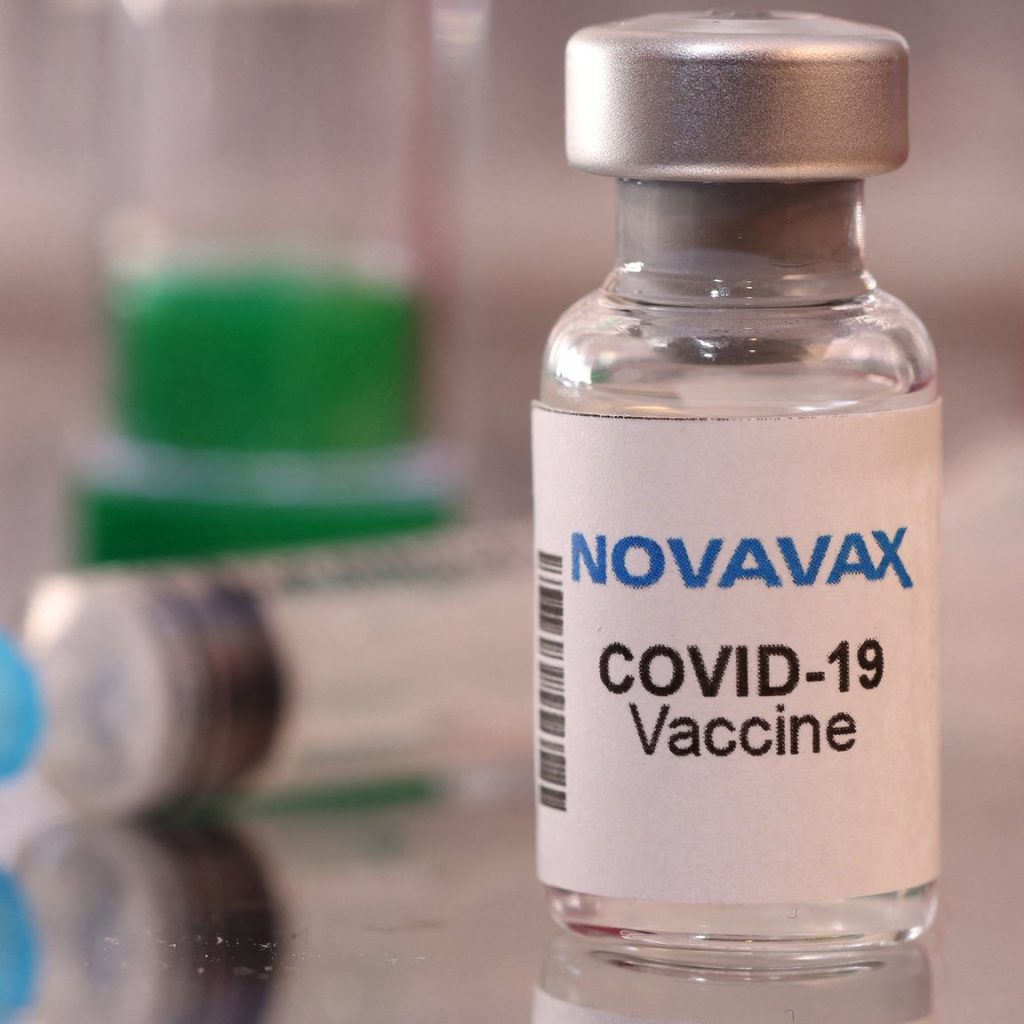
The US government has secured 3.2 million Novavax vaccine doses, but is waiting for the company to finish quality testing before they can be released
Advisers to the US Centers for Disease Control and Prevention (CDC) yesterday voted unanimously to recommend use of Novavax’s COVID-19 vaccine for individuals aged 18 years and above.
The shot has been endorsed by the CDC’s Advisory Committee on Immunization Practices (ACIP). Rochelle Walensky, Director, CDC, still needs to sign off on the recommendations before the vaccine can be made available in the US, and the shot may not be immediately ready for rollout.
The US government has secured 3.2 million Novavax vaccine doses, but is waiting for the company to finish quality testing before they can be released. Novovax hopes to finish testing in the next few weeks.
The vaccine was authorised by the US Food and Drug Administration (FDA) last week as a two-dose primary vaccination series, becoming the fourth COVID shot to be authorised for use in adults in the US.










